Elmiron Lawsuit - IC and Painful Bladder Med Linked to Vision Loss, Eye Disease
The widely used copper IUD known as Paragard may be prone to breakage, migration or other problems occurring shortly after implantation or upon routine removal. These problems can cause serious complications for women who use the birth control device, possibly including organ perforation, scarring inside the uterus and the need for revision surgery to remove the fractured IUD components.
If you or a loved one experienced breakage of our Paragard device during a routine removal, please contact us as soon as possible to discuss the possibility of filing a Paragard lawsuit today.
Reasons to File a Paragard Lawsuit
A growing number of lawsuits accuse the manufacturers of Paragard of:
- Designing a defective and dangerous medical device
- Knowing about the potential risk of side effects from Paragard
- Failing to adequately research the potential complications associated with Paragard
- Misrepresenting Paragard as a safe and effective birth control device
- Failing to warn users about the potential risk of Paragard breakage, migration and other side effects
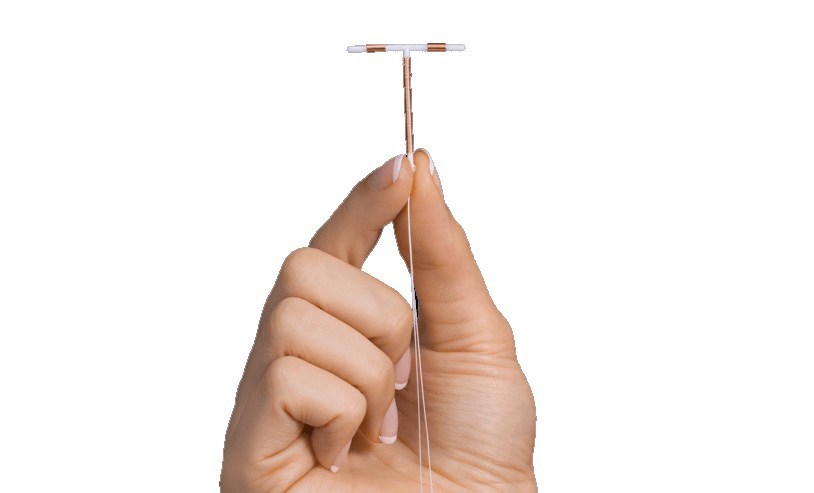
What is Paragard?
"Paragard has been linked to breakage upon routine removal leading to serious health consequences for women who trusted the device."
What is Paragard?
Paragard is a small, T-shaped intrauterine device (IUD) designed to be implanted in the uterus as a long-term, reversible form of birth control. Unlike oral contraceptives, Paragard does not use artificial hormones to prevent pregnancy. Rather, the IUD features a copper wire coiled around a plastic frame that produces an inflammatory reaction that is toxic to sperm and eggs. Paragard was approved by the U.S. Food and Drug Administration (FDA) more than 30 years ago and was originally manufactured by Teva Pharmaceuticals, before being acquired by The Cooper Companies, Inc. in 2017. One of the key benefits of Paragard is that placement is nonsurgical and takes only a few minutes. In fact, it can be done by a healthcare provider during a routine office visit. And once the device is in place, Paragard is meant to provide continuous pregnancy prevention for up to 10 years and can be removed at any time. Unfortunately for many women, using and removing Paragard is not as easy as advertised.
How a Paragard Lawsuit Can Help
The economic and non-economic damages that can arise in a Paragard IUD lawsuit include:
- Medical expenses
- Cost of surgery
- Pain and suffering
- Lost wages due to missed time at work
- Pain and suffering
- Permanent disability or disfigurement
- Loss of consortium
Reports of Problems with Paragard
IUDs are one of the easiest and most efficient birth control methods on the market. They are designed to provide continuous pregnancy prevention for up to a decade and trials show that they are more than 99% effective. For this reason, Paragard and other IUDs have become a popular method of birth control for women looking for a low-maintenance, hormone-free alternative to oral contraceptives. However, serious concerns have been raised about the safety of Paragard IUD, following reports of the device migrating out of position or breaking apart inside of women’s bodies during the removal process. Some women have required surgery to have the broken Paragard pieces removed and others have suffered serious complications like perforation of the uterine wall, organ damage, scarring inside the uterus, inflammation or injury from copper pieces left inside the body, and the possible need for a hysterectomy.
Since 2010, the FDA has received more than 1,600 reports of device breakage and other problems with Paragard IUD. According to reports, defects in the design or manufacturing of Paragard may render the device vulnerable to snapping at the T-joint during the removal process, causing the device to break apart inside the body. If this happens and the physician is unable to locate the broken arm of the IUD, it could become embedded in and cause irreversible damage to the uterus. Other adverse event reports submitted to the FDA have highlighted incidents where women experienced partial or full expulsion of the device, requiring surgery, or had their Paragard IUD removed by their doctor, only to find that the copper coil was missing from the frame.
Did you or a loved one experience breakage of a Paragard IUD birth control device during a routine removal?
You may qualify for significant compensation.
Find Out MoreConsumer Safety Watch offers safety advocate services and attorney referral services for patients throughout the United States including the states of Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin and Wyoming.
Consumer Safety Watch offers consumer safety advocate services or can help you find an attorney throughout the United States including the following cities: Albuquerque, NM; Arlington, TX; Atlanta, GA; Austin, TX; Baltimore, MD; Boston, MA; Charlotte, NC; Chicago, IL; Cleveland, OH; Colorado Springs, CO; Columbus, OH; Dallas, TX; Denver, CO; Detroit, MI; Fresno, CA; Fort Worth, TX; Indianapolis, IN; Honolulu, HI; Houston, TX; Jacksonville, FL; Kansas City, KS; Kansas City, MO; Las Vegas, NV; Long Beach, CA; Los Angeles, CA; Louisville, KY; Memphis, TN; Mesa, AZ; Miami, FL; Miami, OH; Milwaukee, WI; Minneapolis, MN; Nashville, TN; New York City, NY; Oakland, CA; Oklahoma City, OK; Omaha, NE; Philadelphia, PA; Phoenix, AZ; Pittsburgh, PA; Portland, OR; Sacramento, CA; San Antonio, Tx; San Diego, CA; San Francisco, CA; San Jose, CA; Seattle, WA; St. Louis, MO; Tampa, FL; Tucson, AZ; Tulsa, Virginia Beach, VA; Washington, DC; Wichita, KS.
This website is not affiliated with any pharmaceutical or medical device company or any trademarked product. Results are not guaranteed. This website provides a free matching service and is not responsible for information or services from third party providers. Consumer Safety Watch is not a law firm. Every case is different and services available can vary.


