How a BHR Hip Implant Lawsuit Can Help
- Debilitating physical injuries
- Emotional trauma
- Revision surgery
- Medical monitoring and blood tests
- Physical therapy
- Long-term follow-up care
- Lost wages
- Loss of future earnings
Like other all-metal hip implants, Smith & Nephew’s controversial metal-on-metal BHR hip resurfacing system has been plagued by serious problems ranging from device dislocation to joint pain, infection, and metal blood poisoning possibly resulting in the need for revision surgery to remove or replace the failed device. If you or a loved one has suffered injuries from a failed Smith & Nephew BHR hip resurfacing implant, contact an experienced defective hip implant lawyer today to discuss your possible compensation options.
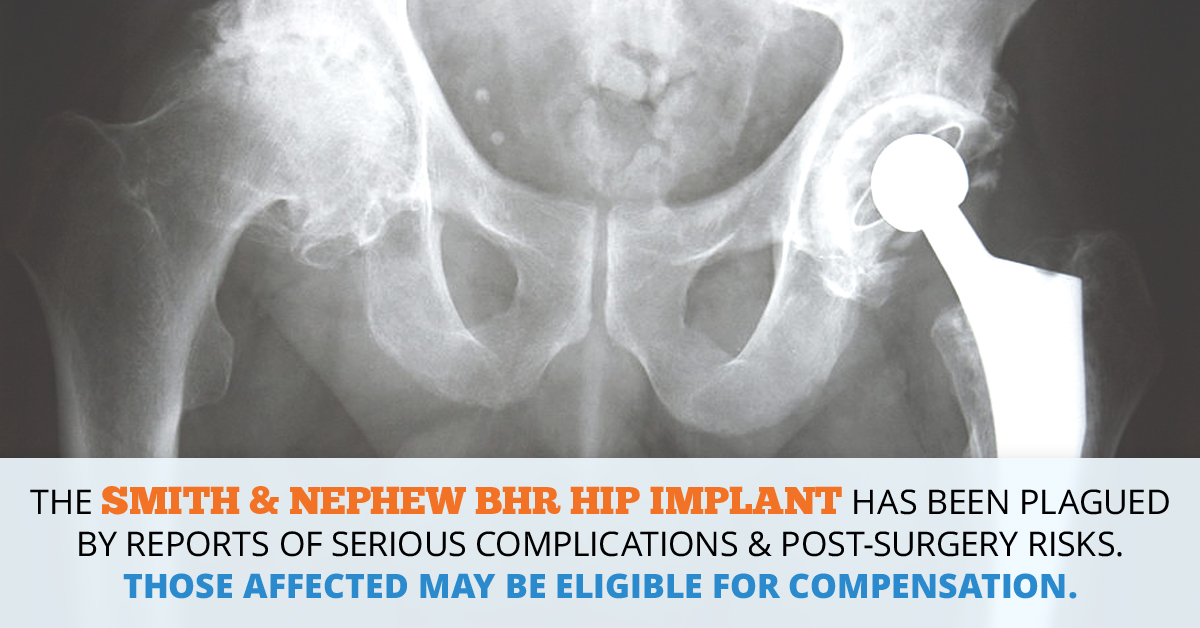
Smith & Nephew hip implants have been the subject of a number of lawsuits filed in recent months, and lawyers across the country are investigating claims involving complications related to the failure of Smith & Nephew artificial hips, including BHR hip implant side effects. The problems linked to the BHR hip implant are similar to the injuries associated with other metal-on-metal hip devices, which have been well-documented, beginning with the 2010 recall of DePuy Orthopaedics’ ASR XL Acetabular System, which was removed from the market after the U.S. Food and Drug Administration (FDA) received hundreds of complaints from implant recipients who suffered pain and other injuries when their devices failed. Since problems with the BHR hip implant system first began to emerge, several lawsuits have been brought against Smith & Nephew, including one claim by a California man who suffered toxic metal poisoning from the implant and required revision surgery, and another by an Illinois woman whose defective implant left her with a grinding sensation in her hip joint and elevated levels of metal in her bloodstream.
In a 2012 study published in The Lancet, researchers found that 8.3% of hip resurfacing implants failed within five years for women over the age of 55.
The Birmingham Hip Resurfacing (BHR) System was approved by the FDA in 2006, and has since been linked to a host of debilitating injuries in patients, including a buildup of toxic levels of cobalt and chromium in the hip joint and bloodstream, which many require painful and costly revision surgery. According to data compiled by the National Joint Registry of England and Wales, the BHR hip resurfacing system has a seven-year revision rate of 11.76 percent, well above the average acceptable failure rate for a device of its kind. Similar data compiled by the National Joint Replacement Registry of Australia in 2015 showed that the BHR system has a 10-year revision rate of 14.5 percent for women.
In addition to problems with cobalt and chromium hip implants shedding toxic metal particles into patients’ bodies, serious concerns have been raised recently about the safety of resurfacing-style hip implants. In a 2012 study published in The Lancet, researchers found that 8.3% of hip resurfacing implants failed within five years for women over the age of 55. In response to their findings, the researchers recommended that “resurfacing procedures [not be] undertaken in women.” Three years later, Smith & Nephew finally issued a hazard alert update, indicating that the BHR system should no longer be used in female patients. The company also withdrew BHR femoral head components sized 46 mm in diameter and smaller from the market, and recommended that patients requiring a 48 mm femoral head size not be considered as candidates for BHR implantation, due to an increased risk of revision.
The Birmingham Hip Resurfacing System is a metal-on-metal hip implant used to preserve, rather than replace, the femoral head and neck in patients requiring a hip arthroplasty, or total hip replacement, due to inflammatory arthritis or degenerative joint disease. Considered a “bone conserving procedure,” the hip resurfacing system consists of a cemented As Cast CoCr (cobalt-chromium) femoral component and an uncemented As Cast CoCr coated acetabular component, and is designed to be an alternative to traditional total hip arthroplasty implants, which typically replace the entire worn-down femur with a prosthetic one. According to the Smith & Nephew website, the BHR implant is designed to decrease the risk of dislocation and facilitate future replacement or revision surgery, and is best suited to younger, more active male patients.
FDA – Class 2 Device Recall Smith Nephew, Birmingham Hip Resurfacing, Acetabular Cup