Reports of Problems with Paragard
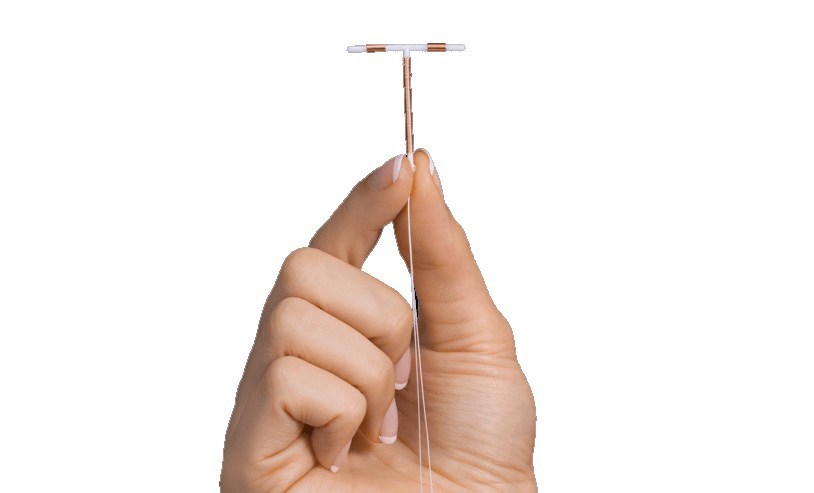
IUDs are one of the easiest and most efficient birth control methods on the market. They are designed to provide continuous pregnancy prevention for up to a decade and trials show that they are more than 99% effective. For this reason, Paragard and other IUDs have become a popular method of birth control for women looking for a low-maintenance, hormone-free alternative to oral contraceptives. However, serious concerns have been raised about the safety of Paragard IUD, following reports of the device migrating out of position or breaking apart inside of women’s bodies during the removal process. Some women have required surgery to have the broken Paragard pieces removed and others have suffered serious complications like perforation of the uterine wall, organ damage, scarring inside the uterus, inflammation or injury from copper pieces left inside the body, and the possible need for a hysterectomy.
Since 2010, the FDA has received more than 1,600 reports of device breakage and other problems with Paragard IUD. According to reports, defects in the design or manufacturing of Paragard may render the device vulnerable to snapping at the T-joint during the removal process, causing the device to break apart inside the body. If this happens and the physician is unable to locate the broken arm of the IUD, it could become embedded in and cause irreversible damage to the uterus. Other adverse event reports submitted to the FDA have highlighted incidents where women experienced partial or full expulsion of the device, requiring surgery, or had their Paragard IUD removed by their doctor, only to find that the copper coil was missing from the frame.